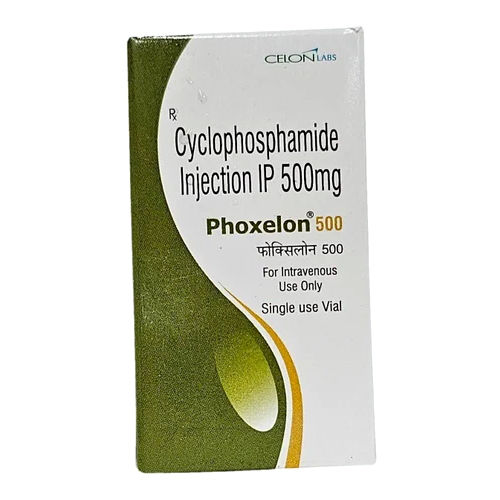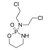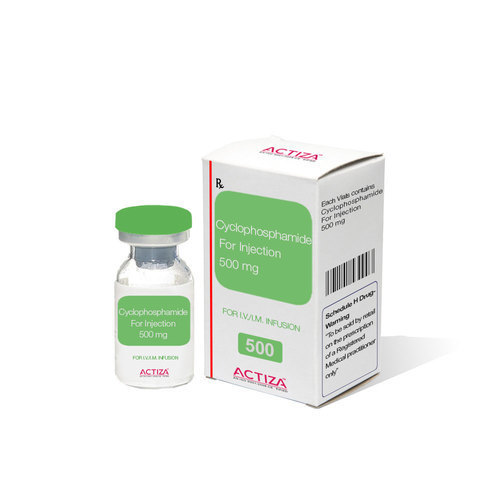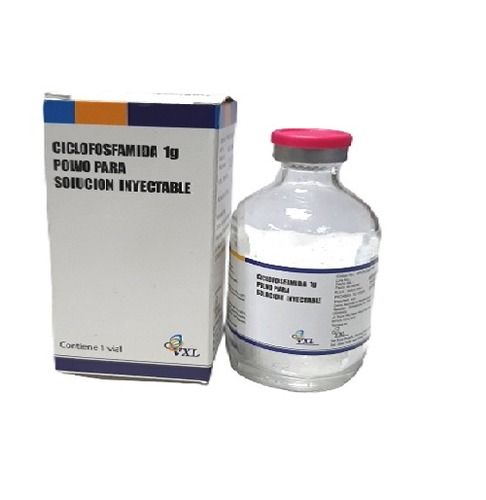
Cyclophosphamide Injection Usp 1 Gm Shelf Life: 3 Years
Price: 150 INR / Piece
Get Latest Price
Minimum Order Quantity :
500 Piece
In Stock
Product Specifications
| Enzyme Types | Other |
| Ingredients | Other |
| Physical Color/Texture | Other |
| Fermentation Smell | Slight Smell |
| Storage Instructions | 30 |
| Shelf Life | 3 Years |
| Supply Ability | 2000 Per Day |
| Delivery Time | 2 Days |
| Sample Available | Yes |
| Sample Policy | If order is confirmed we will reimburse the sample cost |
| Main Export Market(s) | Western Europe, Central America, Middle East, Asia, Africa |
Product Overview
Key Features
Refer to the a Also Known Asa section to reference different products that include the same medication as Cyclophosphamide.
Drug Basics
Brand Name: CYCLOPHOSPHAMIDE INJECTION USP 1 GM
Generic Name: CYCLOPHOSPHAMIDE INJECTION USP 1 GM
Drug Type: HUMAN PRESCRIPTION DRUG
Route: ORAL, INTRAVENOUS
Dosage Form: INJECTION, POWDER, FOR SOLUTION
Indications & Usage
Cyclophosphamide for injection is an alkylating drug indicated for treatment of:
a cMalignant Diseases: malignant lymphomas: Hodgkin's disease, lymphocytic lymphoma, mixed-cell type lymphoma, histiocytic lymphoma, Burkitt's lymphoma; multiple myeloma, leukemias, mycosis fungoides, neuroblastoma, adenocarcinoma of ovary, retinoblastoma, breast carcinoma. (1.1)
a cMinimal Change Nephrotic Syndrome in Pediatric Patients: biopsy proven minimal change nephrotic syndrome patients who failed to adequately respond to or are unable to tolerate adrenocorticosteroid therapy. (1.2)
Limitations of Use:
The safety and effectiveness for the treatment of nephrotic syndrome in adults or other renal disease has not been established.
Malignant diseases
Cyclophosphamide for injection is indicated for the treatment of:
a cmalignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkina s disease, lymphocytic lymphoma (nodular or diffuse), mixed-cell type lymphoma, histiocytic lymphoma, Burkitta s lymphoma
a cmultiple myeloma
a cleukemias: Chronic lymphocytic leukemia, chronic granulocytic leukemia (it is usually ineffective in acute blastic crisis), acute myelogenous and monocytic leukemia, acute lymphoblastic (stem-cell) leukemia (cyclophosphamide for injection given during remission is effective in prolonging its duration)
a cmycosis fungoides (advanced disease)
a cneuroblastoma (disseminated disease)
a cadenocarcinoma of the ovary
a cretinoblastoma
a ccarcinoma of the breast
Cyclophosphamide for injection, although effective alone in susceptible malignancies, is more frequently used concurrently or sequentially with other antineoplastic drugs.
Minimal Change nephrotic Syndrome In Pediatric Patients
Cyclophosphamide for injection is indicated for the treatment of biopsy proven minimal change nephrotic syndrome in pediatrics patients who failed to adequately respond to or are unable to tolerate adrenocorticosteroid therapy.
Limitations of Use:
The safety and effectiveness for the treatment of nephrotic syndrome in adults or other renal disease has not been established.
Adverse Reactions
The following adverse reactions are discussed in more detail in other sections of the labeling.
a cHypersensitivity [see Contraindications (4)]
a cMyelosuppression, Immunosuppression, Bone Marrow Failure, and Infections [see Warnings and Precautions (5.1)]
a cUrinary Tract and Renal Toxicity [see Warnings and Precautions (5.2)]
a cCardiotoxicity [see Warnings and Precautions (5.3)]
a cPulmonary Toxicity [see Warnings and Precautions (5.4)]
a cSecondary Malignancies [see Warnings and Precautions (5.5)]
a cVeno-occlusive Liver Disease [see Warnings and Precautions (5.6)]
a cEmbryo-Fetal Toxicity [see Warnings and Precautions (5.7)]
a cReproductive System Toxicity [see Warnings and Precautions (5.8) and Use in Specific Populations (8.4 and 8.6)]
a cImpaired Wound Healing [see Warnings and Precautions (5.9)]
a cHyponatremia [see Warnings and Precautions (5.10)]
Company Details
Vee Excel Drugs and Pharmaceuticals is a leading Manufacturer and Exporter of various healthcare products. The company has its root back in early 2000, with Pharmaceutical range of products, keeping 100% focus on the international market. Over the period of time, understanding the need to serve various demand of clientele under single roof, the company introduced its Ayurvedic range of products and gradually had added its Veterinary division. Today the company is well known for its endless effort to maintain the quality standard. To keep the company values alive and to serve people with quality medicines, the company had created its own R&D facility in its new manufacturing unit. The new unit of Vee Excel Drugs and Pharmaceuticals, is a state of the art manufacturing facility, with all modern amenities and latest technology. Situated at B1/A, EPIP Site V, Road no. 25, Kasna, Greater Noida, it was built as per European compliance with future prospect of entering into the European market. The new facility is spread at an area 5454 sq.mt has got 3 individual buildings with totally equipped in-house cafeteria and working zones. The building at the entrance has got the R&D lab, and a seperate set up for Oncology, with Tablets, Capsules and Injectables dosage form. The second building is dedicated for the Cephalosporin group with Tablets, Capsules, Dry syrup dosage, and the 3rd building for the General group and Dietary supplements, again in Tablets, Capsules and Dry Syrup. Today, Vee Excel has got a team of approx 200 people comprises of skilled, dynamic, experienced and technically sound professionals. Every team member work cumulatively to achieve the overall objective of the organization, which eventually help the company to successfully enter new market and increasing its customer base.
Business Type
Exporter, Manufacturer, Supplier
Employee Count
200
Establishment
2000
Working Days
Monday To Saturday
GST NO
09AABCV3624M3ZZ
Certification
ISO 9001:2015,ISO 14001:2015; ISO 22000:2018,WHO-GMP;OHSAS 45001;2015
Related Products
Explore Related Categories
Seller Details

GST - 09AABCV3624M3ZZ
Greater Noida, Uttar Pradesh

Accepts only Foreign inquiries
Director Marketing
Mrs Anu Bansal
Members since
6 Years
Address
Plant Address : B No, B-1/A, EPIP Site V, Kasana, Road No. 25, Kasana, Greater Noida, Uttar Pradesh, 201308, India
cyclophosphamide injection in Greater Noida
Report incorrect details