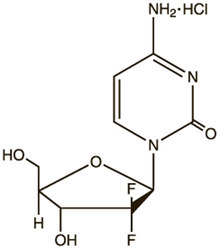
Gemcitabine Hydrochloride Powder For Solution For Infusion Ingredients: Bupivacaine
Price:
Get Latest Price
Minimum Order Quantity :
1
In Stock
Product Specifications
| Ingredients | Bupivacaine |
| Physical Form | Liquid |
| Function | Anti-Cancer |
| Recommended For | All |
| Dosage | As Per Doctor Recommendation |
| Storage Instructions | Dry place |
| Supply Ability | 5000-10000 Per Month |
| Delivery Time | 2 Week |
| Main Domestic Market | All India |
Company Details
GoodFaith Pharma IMPEX is a Pharmaceutical Exports company that aims to deliver Pharma related products to near and far flung locations across the world.
Business Type
Exporter, Manufacturer, Supplier
Employee Count
10
Establishment
2018
Working Days
Monday To Sunday
GST NO
27AASFG6496L1ZD
Payment Mode
Others
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 27AASFG6496L1ZD
Nagpur, Maharashtra

Accepts only Foreign inquiries
Partner
Mr Vinit Kalyani
Address
102, Vidarbha Plaza, New Colony, Nagpur, Maharashtra, 440013, India
gemcitabine injection in Nagpur
Report incorrect details