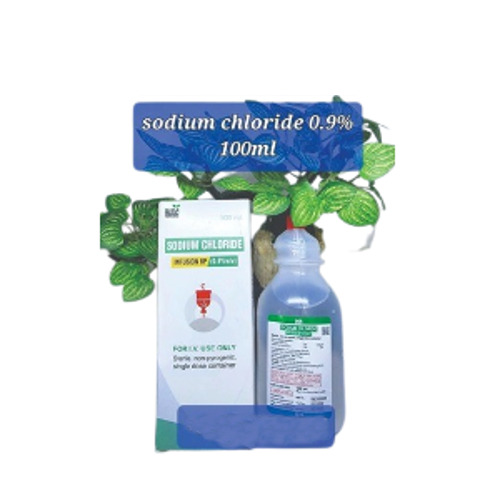
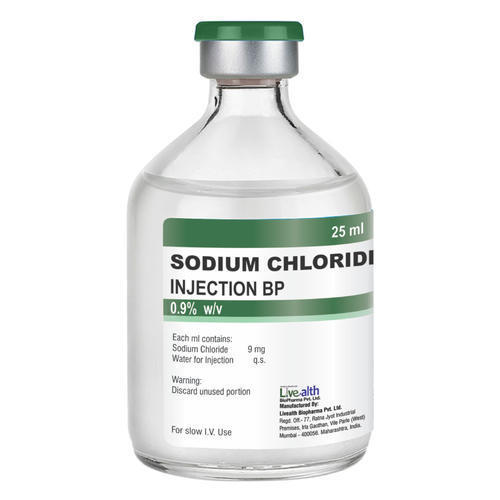
Sodium Chloride Injection Ip 0.9% W/v 500ml (Ns) Liquid
Price: 60 INR / Bottle
Get Latest Price
Minimum Order Quantity :
50000 Bottle
In Stock
Product Specifications
| Dosage Form | Liquid |
| Origin of Medicine | India |
| Pacakaging (Quantity Per Box) | 500ml per bottle |
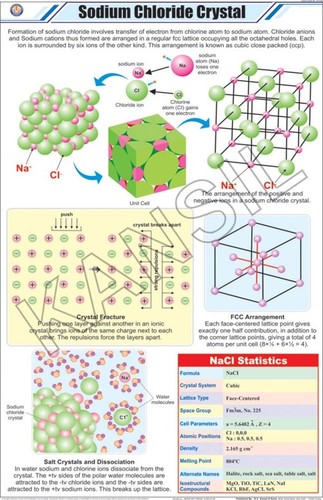
| Salt Composition | Sodium Chloride Injection (NS), IP, BP & USP 0.9% is a formulation of sodium chloride in Water for Intravascular Injection. No preservative, antimicrobial agent or buffer is added. Sodium Chloride Injection, IP, BP & USP 0.9% is provided as a sterile, nonpyrogenic, clear, colorless, odorless solution. Each ml of Sodium Chloride Injection, USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The Osmolarity is 308 mosm/L (calc.). Sodium Chloride Injection, USP 0.9% is designated chemically as sodium chloride and its molecular formula is NaCl. Its molecular weight is 58.44. |
| Indication | Sodium Chloride Injection (NS), IP, BP & USP 0.9% is a formulation of sodium chloride in Water for Intravascular Injection. No preservative, antimicrobial agent or buffer is added. Sodium Chloride Injection, IP, BP & USP 0.9% is provided as a sterile, nonpyrogenic, clear, colorless, odorless solution. Each ml of Sodium Chloride Injection, USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The Osmolarity is 308 mosm/L (calc.). Sodium Chloride Injection, USP 0.9% is designated chemically as sodium chloride and its molecular formula is NaCl. Its molecular weight is 58.44. |
| Drug Type | Injection |
| Formulations Type | Injectables |
| Ingredients | Sodium Chloride Injection (NS), IP, BP & USP 0.9% is a formulation of sodium chloride in Water for Intravascular Injection. No preservative, antimicrobial agent or buffer is added. Sodium Chloride Injection, IP, BP & USP 0.9% is provided as a sterile, nonpyrogenic, clear, colorless, odorless solution.Each ml of Sodium Chloride Injection, USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The Osmolarity is 308 mosm/L (calc.). Sodium Chloride Injection, USP 0.9% is designated chemically as sodium chloride and its molecular formula is NaCl. Its molecular weight is 58.44. |
| Physical Form | Liquid |
| Formulations Form | Liquid |
| Function | Antibiotic Medicine |
| Recommended For | Sodium Chloride Injection (NS), IP, BP & USP 0.9% is a formulation of sodium chloride in Water for Intravascular Injection. No preservative, antimicrobial agent or buffer is added. Sodium Chloride Injection, IP, BP & USP 0.9% is provided as a sterile, nonpyrogenic, clear, colorless, odorless solution. Each ml of Sodium Chloride Injection, USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The Osmolarity is 308 mosm/L (calc.). Sodium Chloride Injection, USP 0.9% is designated chemically as sodium chloride and its molecular formula is NaCl. Its molecular weight is 58.44. |
| Dosage | Sodium Chloride Injection (NS), IP, BP & USP 0.9% is a formulation of sodium chloride in Water for Intravascular Injection. No preservative, antimicrobial agent or buffer is added. Sodium Chloride Injection, IP, BP & USP 0.9% is provided as a sterile, nonpyrogenic, clear, colorless, odorless solution. Each ml of Sodium Chloride Injection, USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The Osmolarity is 308 mosm/L (calc.). Sodium Chloride Injection, USP 0.9% is designated chemically as sodium chloride and its molecular formula is NaCl. Its molecular weight is 58.44. |
| Dosage Guidelines | As per physician |
| Suitable For | Adults |
| Quantity | 500 ml Container |
| Storage Instructions | Store it at room temperature |
| Payment Terms | Cash in Advance (CID) |
| Supply Ability | 500000 Per Week |
| Delivery Time | 30 Days |
| Sample Policy | Sample costs shipping and taxes has to be paid by the buyer |
| Packaging Details | Sodium Chloride Injection (NS), IP, BP & USP 0.9% is packed in infusion and customized packaging is also available. |
| Main Export Market(s) | Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa |
| Main Domestic Market | All India |
| Certifications | WHO, GMP, ISO |
Product Overview
Key Features
Each ml of Sodium Chloride Injection, USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The Osmolarity is 308 mosm/L (calc.). Sodium Chloride Injection, USP 0.9% is designated chemically as sodium chloride and its molecular formula is NaCl. Its molecular weight is 58.44.
Company Details
Founded in the year 2017, We, Facmed Pharmaceuticals Pvt. Ltd. are an emerging pharmaceutical organization with proven capabilities in the area of Manufacturing, Exporting, Trading and Supplying different kinds of pharmaceutical products. We deal in different kinds of Pharmaceuticals Products, Medical Devices, Veterinary Products & Food Supplements, Herbal Products & Nutritional Supplements etc. We apply a unique combination of expertise in the field of healthcare and believe in bringing new ideas for preparing medical products which are very beneficial for health conscious clients.
Business Type
Exporter, Manufacturer, Trading Company
Employee Count
10
Establishment
2017
Working Days
Monday To Saturday
GST NO
07AADCF1478P1Z5
Certification
ISO 9001:2015
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 07AADCF1478P1Z5
New Delhi, Delhi
Bd, Ra & Qa.
Mr. Puneet
Members since
8 Years
Address
S/F, Plot No. B-133-134, Nanhey Park, Kh No 18/20, Uttam Nagar, New Delhi, Delhi, 110059, India
Report incorrect details